Delving into the realm of Pfizer Side Effects and Medication Safety Standards, this paragraph sets the stage for an enlightening journey, captivating readers with intriguing insights and valuable information.
The following paragraphs will provide a detailed exploration of the topic, shedding light on common side effects, safety standards, and more.
Pfizer Side Effects
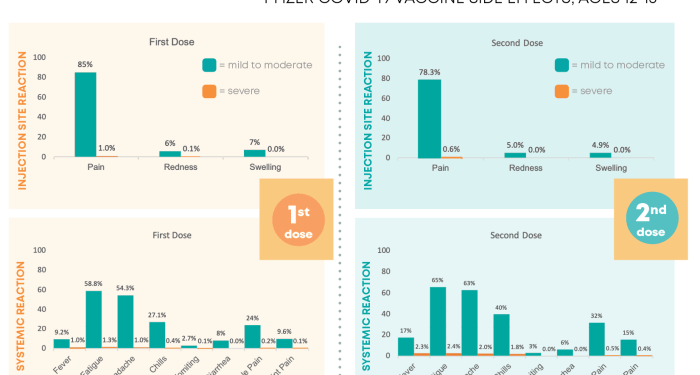
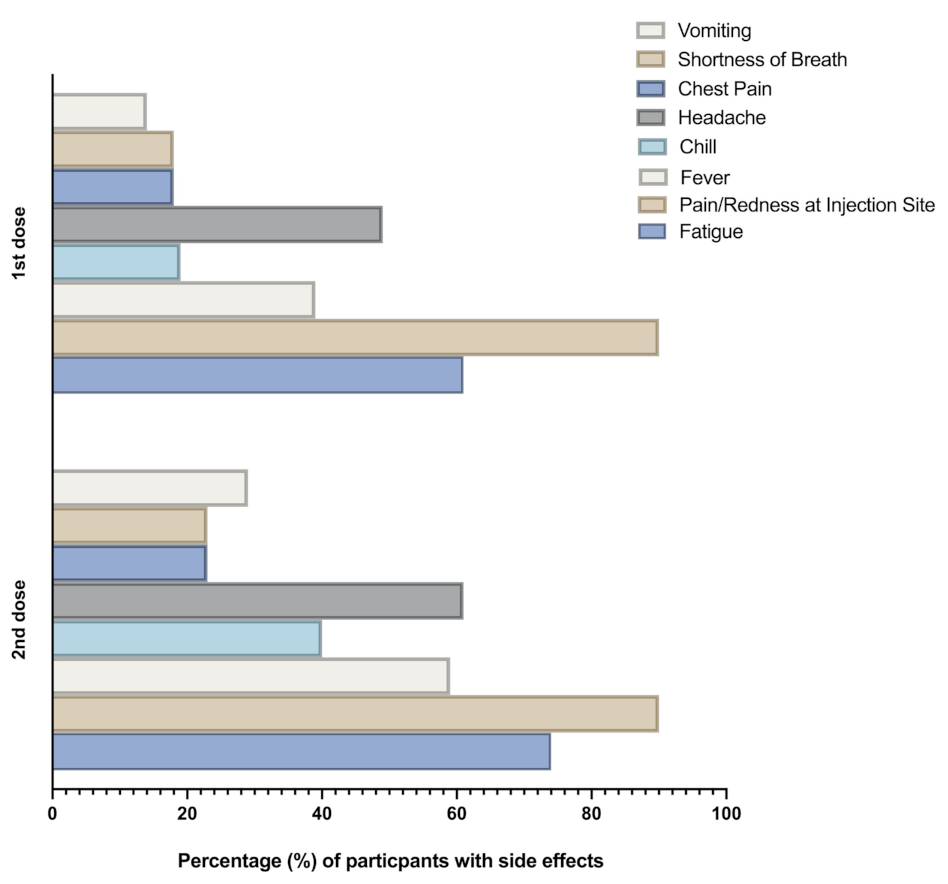
The Pfizer COVID-19 vaccines have been widely used in vaccination campaigns around the world. Like all vaccines, they may cause side effects in some individuals. It is important to be aware of these side effects to ensure safety and well-being.
Common Side Effects
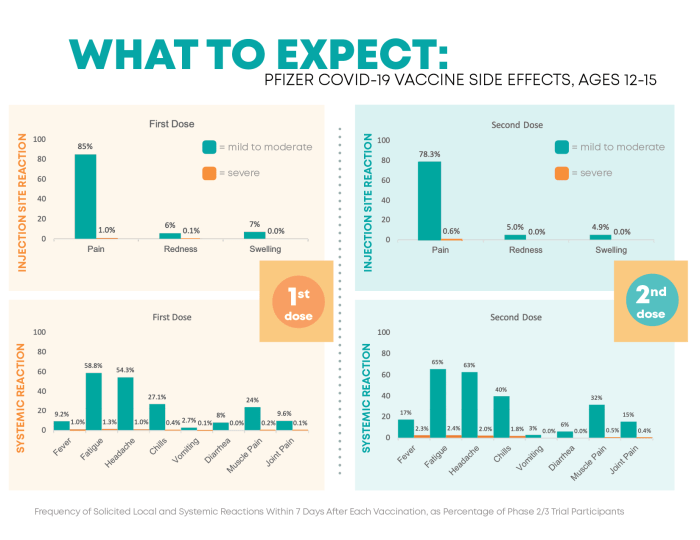
- Fatigue
- Headache
- Muscle pain
- Chills
- Fever
- Injection site pain or swelling
Severe or Rare Side Effects
- Anaphylaxis (severe allergic reaction)
- Myocarditis (inflammation of the heart muscle)
- Thrombosis with thrombocytopenia syndrome (rare blood clotting disorder)
- Guillain-Barré Syndrome (rare neurological disorder)
Importance of Monitoring and Reporting
It is crucial to monitor and report any side effects experienced after receiving the Pfizer vaccine. This helps health authorities and medical professionals track the safety of the vaccine and take appropriate actions if needed.
Comparison with Other COVID-19 Vaccines
Compared to other COVID-19 vaccines, the side effects of the Pfizer vaccine are generally mild and temporary. However, each vaccine may have specific side effects, and individuals should consult healthcare providers for personalized advice.
Medication Safety Standards
Medication safety standards play a crucial role in ensuring the development and distribution of safe and effective vaccines
Role of Regulatory Bodies
Regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe play a key role in establishing safety standards for medications. These agencies review clinical trial data, manufacturing processes, and safety measures to ensure that medications, including vaccines, meet rigorous safety and efficacy standards before they are approved for use.
Safety Measures by Pfizer
Pfizer, like other pharmaceutical companies, implements various safety measures to ensure the safety of its medications, including vaccines. Some of these measures include rigorous clinical trials involving thousands of participants, ongoing monitoring of adverse events post-approval, and compliance with Good Manufacturing Practices (GMP) to ensure quality and consistency in production.
Impact on Public Trust
Adherence to medication safety standards not only protects public health but also plays a crucial role in building and maintaining public trust in vaccines. When regulatory bodies enforce strict safety standards and pharmaceutical companies like Pfizer adhere to these standards, the public can have confidence in the safety and effectiveness of vaccines, leading to higher vaccination rates and better control of infectious diseases.
Ultimate Conclusion
Wrapping up our discussion on Pfizer Side Effects and Medication Safety Standards, this paragraph offers a comprehensive recap of key points discussed, leaving readers with a deeper understanding of the subject matter.
Helpful Answers
What are some common side effects associated with Pfizer vaccines?
Common side effects include pain at the injection site, fatigue, headache, muscle pain, chills, fever, and nausea. These are usually mild and resolve on their own.
How do Pfizer vaccines compare to other COVID-19 vaccines in terms of side effects?
Pfizer vaccines generally have similar side effects to other COVID-19 vaccines, such as Moderna. However, the severity and frequency of side effects can vary from person to person.
What safety measures does Pfizer implement to ensure medication safety?
Pfizer implements rigorous testing, quality control procedures, and post-market surveillance to ensure medication safety. They also adhere to regulatory guidelines set by health authorities.